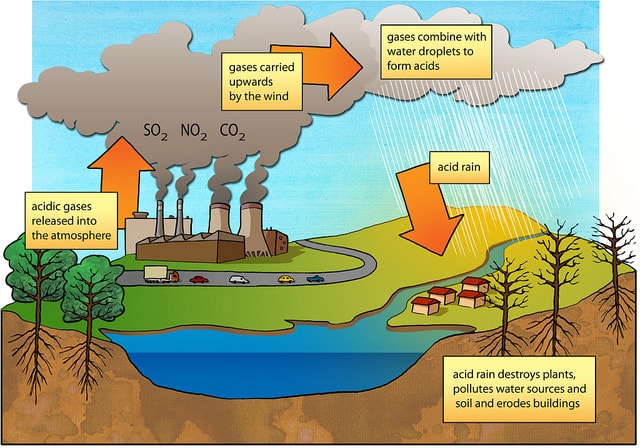
Increases the acidity levels of rivers lakes and seas. Wet deposition causes erosion that affects ecosystems.
What Are The Main Causes Of Acid Rain In Environmental Chemistry By Kakali Ghosh Teacher Blogger M Sc Chemistry Medium
Causes of Acid Rain.
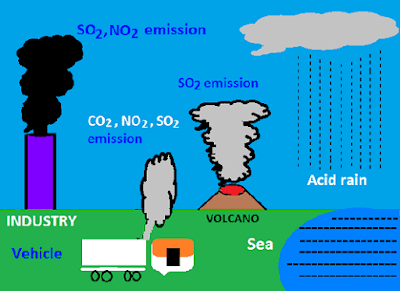
. Volcanoes emit acid-producing gases mainly sulfur to create higher than normal amounts of acid rain or any other form of precipitation such as fog or snow to an extent of affecting vegetation cover and health of residents within the surrounding. Later on these gases emitted through volcano eruptions cause a. The US Environmental Protection Agency EPA reports that that the major contributors to acid rain are SO2 and NOx which result from the burning of oil coal natural gas and other fossil fuels.
Has been found to destroy the roots and leaves of forests in Germany and Scandinavia have been destroyed as the result of acid rain emissions from the UK. Sulfur and Nitrogen Oxides- these are formed by fossil fuels 4 primary fossil fuels are coal-oil-gasoline-and natural gas. Acid rain increases the acidity levels of soils.
Which of the following are natural causes of acid rain. Sulfer Dioxide and Nitrogen Oxide pollute the air and create acid rain. The volcanic eruption is one of the major natural causes of acid rain.
Acid deposition can be caused by natural sources such as volcanoes but it is mainly caused by the release of sulfur dioxide and nitrogen oxide during fossil fuel combustion. These particles will have a pH level below 56. Sulfur and Nitrogen particles which get mixed with water are found in two ways either man-made as the emissions are given out from industries or by natural causes.
This can kill aquatic life. Acid Rain-Download PDF Here. Sulphur and Nitrogen particles which get mixed with water are found in two ways either man-made ie as the emissions that are given out from industries or by natural causes like lightning strike in the atmosphere releasing nitrogen oxides and volcanic.
Get more UPSC MCQs on Environment in the link. Ozone and carbon dioxide. The causes of acid rain are Sulphur and Nitrogen particles which get mixed with the wet components of rain.
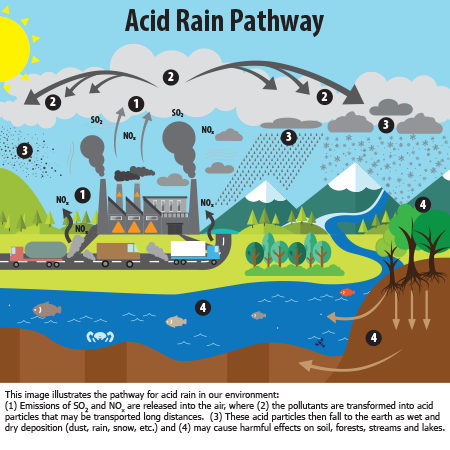
The sulfuric and nitric acids formed in the atmosphere fall to the ground mixed with rain snow fog or hail. What types of pollution cause acid rain. As explained by the EPA acid rain is the result of sulfur dioxide SO2 and nitrogen oxides NOX being emitted into the atmosphere and subsequently transported by wind and air currents.
Acid rain or acid deposition is a broad term that includes any form of precipitation with acidic components such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. When these gases react with the moisture in the atmosphere sulfuric and nitric acids form. Wet deposition is what we most commonly think of as acid rain.
Carbon dioxide and nitrogen. The acidic particles and gases may deposit to surfaces water. The three-carbon molecules broken down from six-carbon molecules of glucose during the first step in the process of nutrition in all organisms is called.
Acid rain describes any form of precipitation that contains high levels of nitric and sulfuric acids. Wind may transport these acid particles over long distances before they come down as wet or dry deposition. When volcanoes erupt it releases a huge amount of acid rain causing gases in the atmosphere such as sulfur.
Natural causes of acid rain are oxides of sulfur and nitrogen from volcanoes swamps and. What causes acid rain. The causes of acid rain are Sulfur and Nitrogen particles which get mixed with the wet components of rain.
These substances can rise very high into the atmosphere where they mix and react with water oxygen and other chemicals to form more acidic pollutants known as acid rain. Previous Year Question on Acid Rain UPSC 2011 Acid rain is caused by the pollution of the environment by. Carbon monoxide and carbon dioxide.
Dry deposition occurs when small acid particles and gases fall onto the earths surface. It can also occur in. Acid rain is wet deposition.
Scientists believe that these natural sources are responsible for one third of the nitrogen and sulfur emissions in the US. Nitrous oxide and Sulphur dioxide. Acid rain causes a range of problems.
Volcanic eruptions release water vapor and gases such as carbon dioxide and monoxide sulfur dioxide hydrogen chloride hydrogen sulfide hydrogen fluoride ammonia methane and silicon. The main natural causal agent for acid rain is volcanic emissions. This can kill vegetation.
This can include rain snow fog hail or even dust that is acidic. Acid rain is caused primarily by sulfur dioxide SO2 and nitrogen oxide NOx emissions. When these gases are discharged into the atmosphere they react with the water oxygen and other gases already present there to form sulfuric acid ammonium nitrate.
Sources of Acid Rain Acid rain is caused by a chemical reaction that begins when compounds like sulfur dioxide and nitrogen oxides are released into the air. Causes of acid rain The precursors or chemical forerunners of acid rain formation result from both natural sources such as volcanoes and decaying vegetation and man-made sources primarily emissions of sulfur dioxide SO 2 and nitrogen oxides NO x resulting from fossil fuel combustion. Carbon dioxide increases from death of trees and causes global warming and atmospheric pollution Air pollution can damage trees crops other plants lakes and animals.
There are two types of deposition processes. What Causes Acid Rain. Acidic particles and gases can also deposit from the atmosphere in the absence of moisture as dry deposition.
Sulfur dioxide and nitrogen oxides dissolve very. Acid rain is formed by pollutants in the air mixing with wet or dry particles. Precipitation collects acidic particles and gases and becomes acidic.
Primary Causes Of Acid Rain Earth Eclipse

What Is Acid Rain Acid Rain Us Epa

Acid Rain Causes Effects And Solutions All American Environmental
0 Comments